Astrocytes
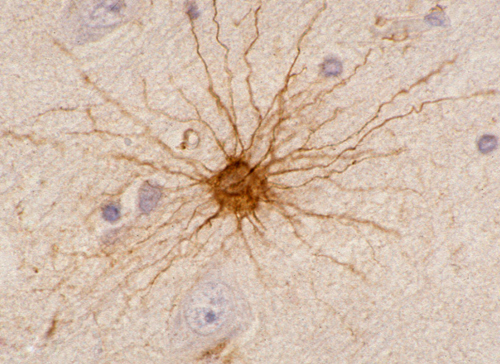
Astrocytes (star cells) have radially arranged processes. Their cytoplasm contains intermediate filaments composed of a distinct protein, glial fibrillary acidic protein (GFAP), encoded by a gene on 17q21. GFAP is also found in nonmyelinating Schwann cells and glial cells of the enteric nervous system. Antibodies against this protein are routinely used to demonstrate reactive and neoplastic astrocytes. Historically, GFAP was the first immunostain to be used. During brain development, astrocytic processes (radial glia) guide neurons in their migration from the wall of the ventricles to the cortex. Radial glia are also a source of neuronal and astrocytic stem cells in the developing brain.
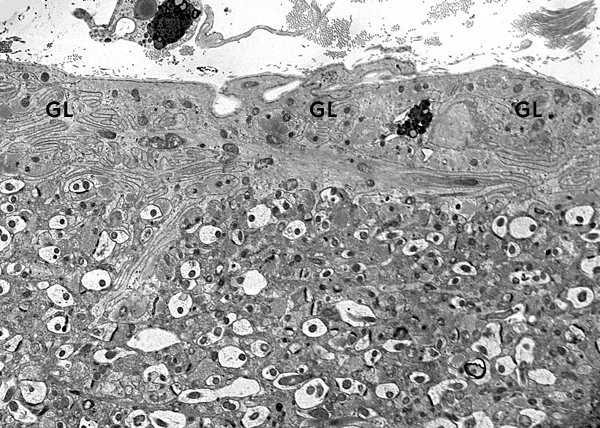
GFAP is a structural protein that maintains the shape and supports the mechanical strength of astrocytes. In the mature brain, there are 5 to 10 times as many astrocytes as there are neurons. The network of astrocytic processes forms the infrastructure on which all other CNS cells and vessels are anchored. Astrocytic processes are intertwined with neurons, axons, and myelin; they cover dendrites and synapses; they surround brain capillaries; interdigitating astrocytic processes form a thick layer on the surface of the CNS, the glia limitans. This layer seals the surface of the CNS and dips into brain tissue along the perivascular (Virchow-Robin) spaces.
Astrocytic foot processes surround brain capillaries and, during development, induce endothelial cells to form tight junctions. The endothelial tight junctions are the basis of the blood-brain barrier, a system of controlled transcapillary transport which maintains homeostasis in the CNS. Endothelial tight junctions are found only in brain capillaries. Loss of the integrity of the endothelial barrier causes fluid to leak into the interstitial space, leading to vasogenic cerebral edema. This raises intracranial pressure and can collapse brain capillaries, resulting in arrest of cerebral perfusion. Cerebral edema is caused by a variety of pathological processes, including ischemic insults, inflammation, and malignant brain tumors whose capillaries do not have tight junctions. Astrocytes are less vulnerable than neurons to ischemic injury but they are damaged if there is lactic acidosis. Such damage causes intracellular fluid accumulation (cytotoxic edema). Cytotoxic edema involves the cerebral cortex, whereas vasogenic edema is more pronounced in the white matter. Vasogenic edema is more important clinically than cytotoxic edema.
Through their extensive contacts and interactions with neurons and vessels astrocytes play a very important role in the function of the CNS in addition to providing structural support. They take up K+ that is released during neuronal activity thus maintaining ion balance in the extracellular fluid. The small amount of glycogen that is present in astrocytes is the only form of stored energy in the CNS and can be used when neuronal activity demands it. More important, the coupling of astrocytes to synapses and vessels facilitates the entry of glucose into the CNS in response to neuronal activity. They take up and recycle GABA and glutamate that are released at synaptic clefts. Glutamate is converted to glutamine by glutamine synthetase. Glutamine is then released into the extracellular space, taken up by neurons, and converted to glutamate by glutaminase. Astrocytes also produce new glutamate from glucose via the tricarboxylic acid cycle. This replenishes glutamate lost through oxidative degradation. Without astrocytes, neurons would not have their most important neurotransmitter. In addition, astrocytes influence synaptic activity by releasing glutamate directly into the extracellular space.
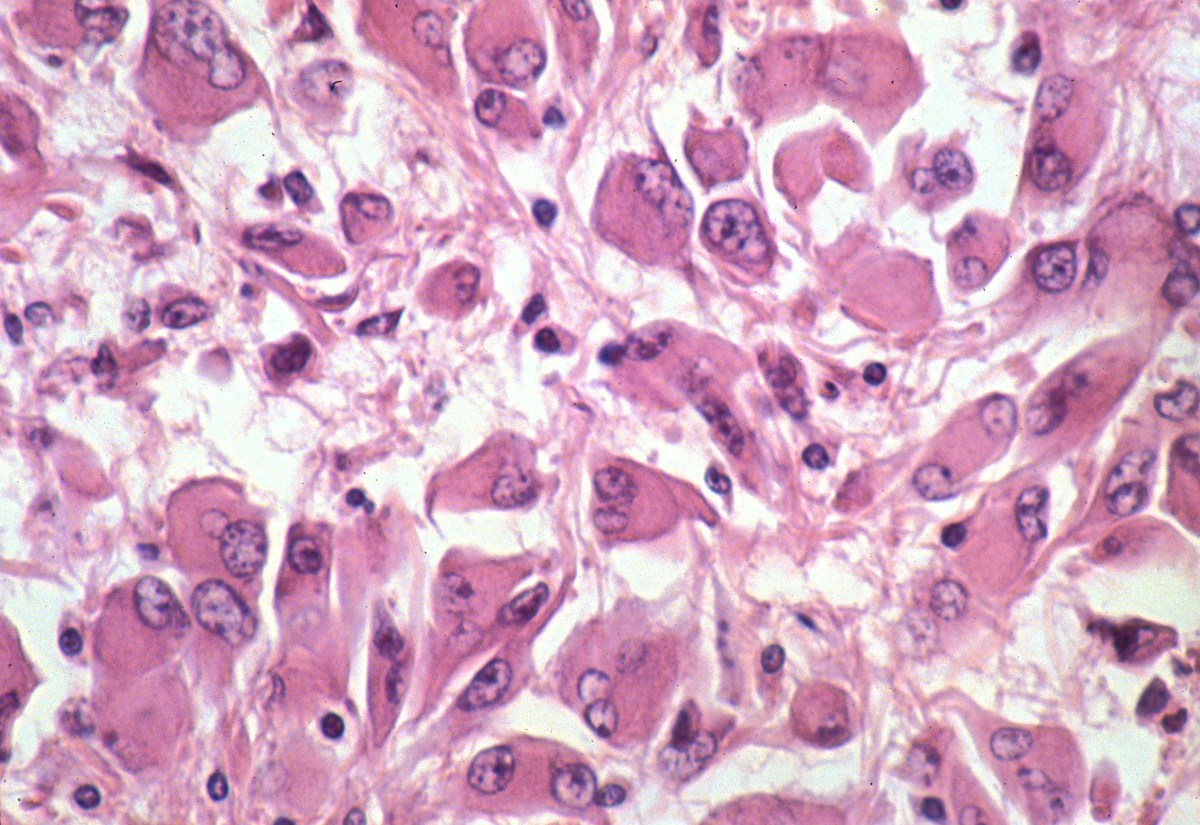
When neurons are lost and brain tissue is damaged from whatever cause, astrocytes proliferate, fill the gaps, and restore CSF-brain and blood-brain barriers. This process, which is called astrogliosis or plain gliosis, involves proliferation and hypertrophy of astrocytes and upregulation of GFAP expression, and is for the CNS what scarring is for extraneural tissues. Moreover, in addition to gliosis, astrocytes participate in inflammatory and degenerative processes by secreting cytokines and assuming various other functions. Some of these functions are neuroprotective and others may be detrimental. Astrocytes participating in gliosis are referred to as reactive astrocytes. They have a large cytoplasmic mass, long, branching processes, and increased cytoplasmic filaments. Such astrocytes are also known as gemistocytic astrocytes from a Greek word that means to fill up. Hepatic failure and other situations associated with high blood ammonia such as porto-systemic shunts precipitate the syndrome of hepatic encephalopathy, characterized by confusion, drowsiness, stupor or coma. Ammonia taken up by astrocytes is converted to osmotically active glutamine, which causes cytotoxic astrocytic swelling. In addition to the rise in intracranial pressure, this process compromises astrocytic function. Swollen astrocytes in hepatic encephalopathy are called Alzheimer type II astrocytes. Their nuclei are large and appear clear in H&E stains. A similar change develops in hypoxia-ischemia. Aquaporin4 (AQP4), the most abundant water channel in the CNS, is located in astrocytic membranes facing the blood-brain and brain-CSF interfaces. In neuromyelitis optica (NMO), IgG autoantibodies elicit an inflammatory reaction that destroys AQP4, leading to disruption of the blood-brain barrier, oligodendrocyte loss, and demyelination. Antibodies to GFAP are responsible for autoimmune GFAP astrocytopathy, a rare condition characterized by meningitis, encephalitis, and myelitis or combinations of these.
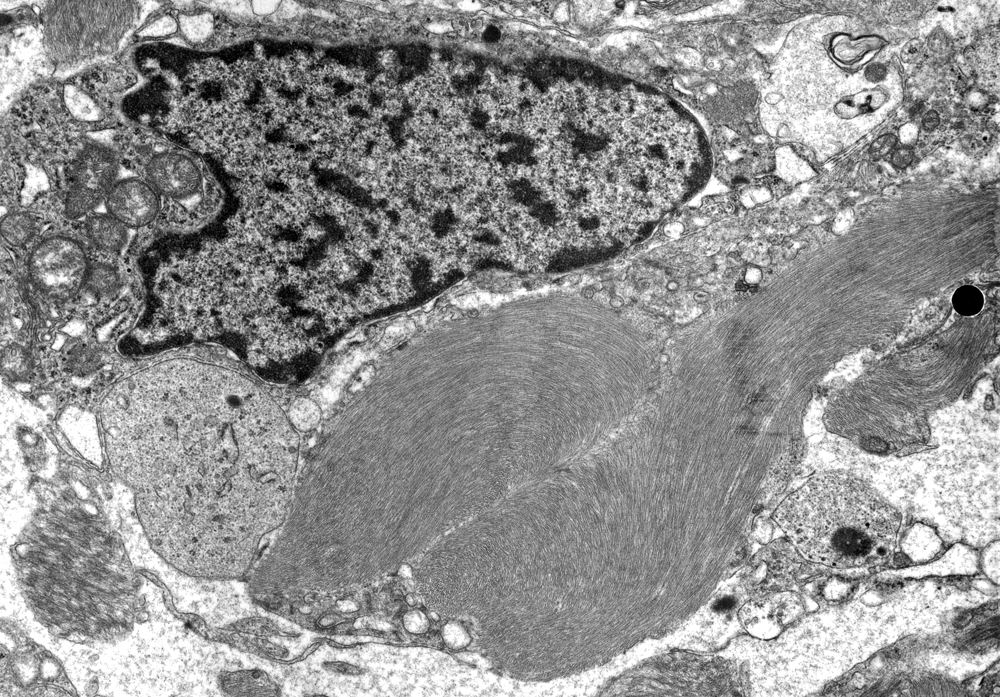
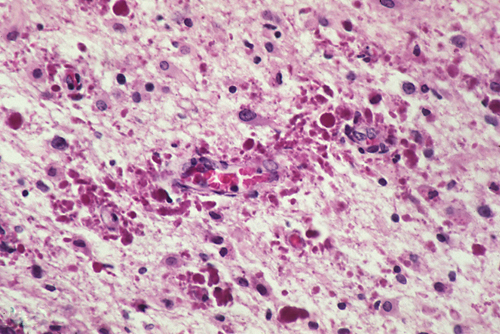
Rosenthal fibers (RFs) are
homogeneous, eosinophilic, elongated,
or globular inclusions in astrocytic
processes. They have a filamentous
and granular structure and contain
GFAP. They are seen in old brain
scars dating back to childhood,
and in some low-grade astrocytomas.
Mutations of GFAP cause Alexander
disease, characterized
by diffuse deposition of RFs,
resulting in white matter degeneration
and neurological dysfunction.
The RFs in Alexander disease
contain mutant GFAP and other
proteins. Corpora
amylacea are spherical intracytoplasmic
bodies of carbohydrate polymers
that develop in astrocytic processes
with advancing age. They have
no pathological significance.
The astrocyte is the cell in
the adult mammalian brain most
capable of undergoing mitosis.
Most brain tumors are derived
from astrocytes (astrocytomas).
Further Reading
- Benarroch EE. Neuron-Astrocyte Interactions: Partnership for Normal Function and Disease in the Central Nervous System. Mayo Clin Proc 2005;80:1326-38. PubMed
- Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends in Neurosciences 2008;31:653-59. PubMed
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships:for better and for worse. Trends in Neurosciences 2011;34:76-87. PubMed
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010 Jan;119(1):7-35. PubMed
- Ratelade J, Verkman AS. Neuromyelitis optica: Aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol. 2012;44(9):1519-30. PubMed
- Yang Z, Wang KKW. Glial fibrillary acidic protein:from intermediate filament assembly and gliosis to neurobiomarker. Trends in Neurosciences 2015;38 (8):364-374. PubMed
- McKeon A, Benarroch EE. Glial fibrillary acid protein. Functions and involvement in disease. Neurology 2018; 90:925-30. PubMed
Updated: August, 2018