Lymphocytes, Monocytes-Macrophages, and Microglia
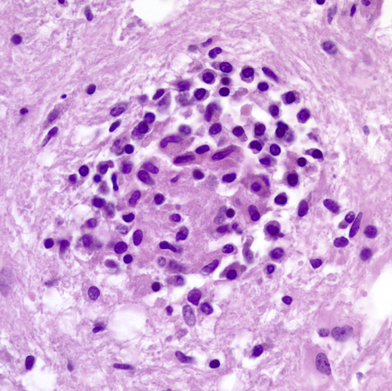
The brain contains very few lymphocytes compared to other organs. In absence of pathology, a few T-lymphocytes regularly cross the intact blood brain barrier, wander in brain tissue for short distances, and re-enter the circulation.(See also the the blood-brain ) Activation of T-lymphocytes as a result of nonspecific infections greatly enhances their ability to randomly cross the blood-brain barrier. Recognition of antigens in CNS parenchyma results in retention and recruitment of T-lymphocytes, leading to inflammation. B-lymphocytes probably also randomly traffic through the brain in a similar fashion and, if they find specific antigens, they aggregate and produce IgG antibodies.
The brain contains two sets of monocyte-macrophage cells, parenchymal microglial cells and blood-borne monocytes. The parenchymal microglial cells are mesodermally derived cells which enter the brain early in gestation. They comprise approximately 12% of cells in the brain and are more abundant in gray matter. Their first function is to clear apoptotic neurons and remove nonfunctioning synapses during development. In the mature brain, they are a very stable and long-lived cell population and are replenished from bone marrow cells at a very low rate. In their resting form, they are inconspicuous. They have elongated nuclei and a small amount of cytoplasm with short processes. Microglial cells have receptors that enable them to sense damaged tissue and to recognize viruses, environmental and endogenous toxins, and other pathogens. Such recognition leads to upregulation (activation) of microglial cells. Activated microglial cells have large rod-shaped nuclei and greatly ramified cytoplasm. They encircle degenerating neurons (neuronophagia) and form clusters around small foci of necrotic brain tissue (microglial nodules). They can also transform into brain macrophages. Activated microglial cells produce trophic factors that are important for neuronal recovery but also make cytokines and neurotoxins such as NO and glutamate that mediate neuroinflammation and can kill neurons. Microglial cells are important for monitoring the CNS environment and restoring homeostasis after CNS injury. However, persistent activation of microglia has damaging effects and is thought to contribute to the neurodegeneration that occurs in Alzheimer's disease, Parkinson's disease, HIV encephalopathy, and other conditions. Microglial activation also develops progressively with advancing age in absence of stimulation.
Macrophages Macrophages in a cerebral infarct. Large cells with a foamy or granular cytoplasm, full of lipid and other products they have ingested. |
Blood-borne monocytes are located in the perivascular spaces, the leptomeninges, and the choroid plexus. The most important of these cells are the perivascular monocytes which reside just outside the vascular basement membrane. These cells are the main antigen-presenting cells of the CNS, thus playing an important role in immune reactions involving the brain. Their location at the interface between brain parenchyma and the vascular system and their continuous circulation in and out of blood vessels suits them ideally for this function. They, along with CD4 lymphocytes, are also the main cells that bring HIV into the brain. Perivascular monocytes can also transform into macrophages. The earliest macrophages following brain injury arise from parenchymal microglia. After a few days, most macrophages arise from perivascular monocytes. Regardless of their derivation, macrophages are large amoeboid cells with a foamy or granular cytoplasm, full of lipid and other products they have ingested.
Tissue Patterns
Neurons and glial cells are arranged in varying patterns in different parts of the CNS. In the cerebrum and cerebellum, the cell bodies and dendrites of neurons are on the surface (cerebral cortex: shell) and their myelinated axons (white matter) in the internal part of the hemispheres. The spinal cord has the opposite design: the neuronal cell bodies (anterior and posterior horns) are in the center and the white matter at the periphery. The white matter of the cerebral hemispheres and spinal cord consists of myelin, oligodendroglia, astrocytes, microglia, and blood vessels. It contains no neurons. The central portion of the cerebral hemispheres (basal ganglia - diencephalon) and the brainstem contain several masses of neurons (nuclei) that are intimately mixed with white matter.
In most of the cerebral cortex (neocortex), neurons are arranged in five layers with a sixth layer consisting of synapses on the surface. These five layers contain mainly two types of neurons, pyramidal cells (large neurons with an apical dendrite and a long axon) and stellate or granule cells (smaller neurons with many dendrites and short axons). Pyramidal cells project their axons to targets away from their points of origin. Stellate cells receive afferents and form intracortical networks. Pyramidal cells are found in layers 3, 5, and 6 and stellate cells mainly in layers 2 and 4. The mix of pyramidal and stellate cells varies in different cortical fields and correlates with cortical function.
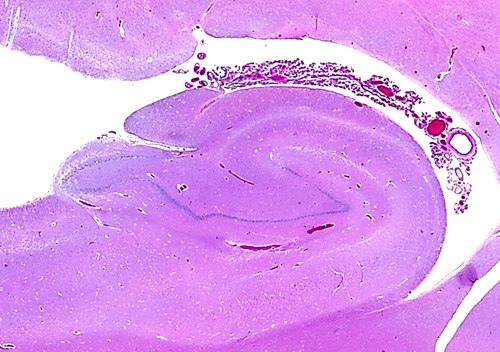
In the medial edge of the temporal lobes, the neuronal layers are reduced to two, the pyramidal and the granular (or dentate gyrus). These layers are wound around one another and form a structure that is called hippocampus (sea horse, literally caterpillar horse), because of its peculiar structure. The two neuronal layers can be best appreciated in coronal sections. The term Ammon's horn, used also for the hippocampus, describes its longitudinal shape, parallel to the temporal lobe. The subiculum and entorhinal cortex are located between the hippocampus and the temporal neocortex. The hippocampus receives extensive afferents from association cortex and limbic areas, and projects to the thalamus, hypothalamus, and cortex. It is especially important for the process of episodic memory, i.e. the memory for personally experienced events. Its role in semantic memory (memory for facts) is not clear and it is not a storage depot for old memories. These are stored at multiple sites in the neocortex. Bilateral lesions of the hippocampus (and medial dorsal nucleus of the thalamus) cause Korsakoff's amnesia, a disorder characterized by inability to remember new things with relative preservation of established memories.
Further Reading
- Block ML, Zecca , Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neuroscience 2007;8:57-69. PubMed
- Prinz M, Jung S, Priller J. Microglia Biology: One Century of Evolving Concepts. Cell 2019;179:292-311.PubMed