PARKINSON'S DISEASE
Parkinson's disease (PD) is the second most common neurodegenerative disease after AD, and the most frequent subcortical degenerative disease. It affects 1-2% of persons older than 60 years. It begins usually in the sixth decade and is characterized by motor symptoms (rigidity, tremor at rest, slowness of voluntary movement, stooped posture, a shuffling, small-step gait, difficulty with balance), and nonmotor symptoms (expressionless face, soft voice, olfactory loss, mood disturbances, dementia, sleep disorders, and autonomic dysfunction, including constipation, cardiac arrhythmias, and hypotension). Constipation, in particular, affects 24.6% to 63% of PD patients and may appear before the onset of motor manifestations.
The vast majority of PD cases are sporadic and are probably caused by interaction of environmental and genetic factors. Approximately 5-10 % of patients have a monogenic, autosomal dominant or recessive form of PD. The first autosomal dominant form of PD identified is that caused by mutations of α-synuclein, a synaptic protein encoded by SNCA on 4q22, which regulates dopamine levels. This discovery led to identification of α-synuclein in Lewy bodies and Lewy neurites (see below). The gene most commonly associated with autosomal recessive PD is Parkin.
Patients with GAucher disease (GD), including carriers of GBA1 mutations, have a 20- to 30-fold risk for developing PD, and 7-10% of PD patients have GBA1 mutations. The clinical and pathological phenotype of PD in GD is similar to idiopathic PD except that it has an earlier onset and causes more cognitive impairment. The mechanism by which GD causes PD is unknown but it is thought to be a combination of lysosomal impairment, mitochondrial dysfunction, oxidative and endoplasmic reticulum stress induced by GD. These changes impair α-synuclein degradation, leading to its accumulation.
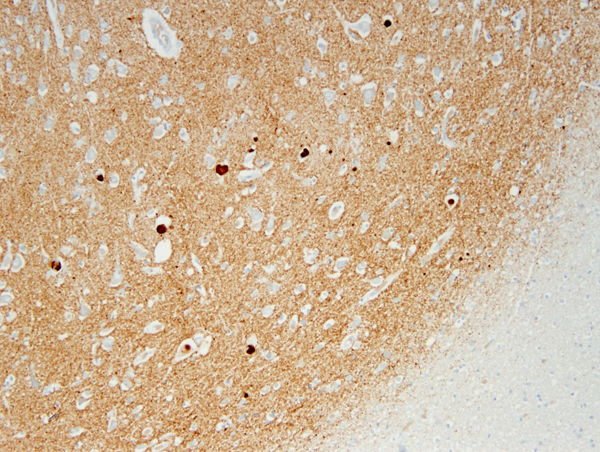
The key pathology in PD is α-synuclein accumulation. Large α-synuclein aggregates form round lamellated eosinophilic cytoplasmic inclusions, Lewy bodies (LBs), in the neuronal body, and fibrils made of insoluble polymers of a-synuclein (Lewy neurites) are deposited in neuronal processes and in astrocytes and oligodendroglial cells. Accumulation of α-synuclein impairs the functions of mitochondria, lysosomes, and endoplasmic reticulum, and interferes with microtubular transport.
The core pathology of PD affects the dopamine-producing neurons of the substantia nigra (SN). These neurons have long and highly branched axons, a large number of synapses and high energy requirements. The mid-section of the SN (zona compacta) is involved earliest and most severely. In advanced PD, loss of pigmented neurons results in gross depigmentation of the SN. Neuromelanin released from dying neurons is picked up by macrophages and astrocytes, and a small amount is found free in the neuropil. Dopamine is produced by SN neurons from DOPA (also a precursor of melanin) and transported along the axons of these neurons to the striatum. The triad of rigidity, bradykinesia and tremor at rest correlates with degeneration of the dopaminergic nigrostriatal pathway and dopamine depletion in the striatum. A-synuclein deposits and LBs are also present in the cerebral cortex, limbic system, and in many extra-nigral neuronal groups, including the reticular activating system, locus ceruleus, dorsal motor nucleus of the vagus, the nucleus basalis of Meynert, olfactory nerves, sympathetic ganglia, and myenteric plexus, leading to impairment of noradrenergic, serotoninergic, and cholinergic neurotransmission. This widespread pathology has only been fully appreciated in recent years, when antibodies to α-synuclein made it easier to detect LBs and synuclein deposits outside the SN. Thus, PD should be viewed as a systemic disorder that affects the entire CNS and PNS, not just the dopaminergic system. This widespread involvement explains the diverse motor and nonmotor manifestations of PD. Although they are characteristic of PD, LBs may be found nonspecifically in many other neurodegenerative conditions.
AD and PD (and in a broader sense tauopathies and synucleinopathies) overlap clinically and pathologically. Extrapyramidal signs develop in AD patients and dementia affects PD patients more frequently than age matched controls. Also, LBs are found in the brains of AD patients, and a large proportion of PD patients have enough SPs and NFTs to qualify for the pathological diagnosis of AD. Until recently, dementia in PD had been attributed primarily to concomitant AD. Recent studies show that cognitive decline in PD correlates better with LBs present in the hippocampus, amygdala, and neocortex.
The treatment of PD consists mainly of L-dopa given with a DOPA decarboxylase inhibitor. An additional therapy for PD patients whose motor symptoms respond to but are not optimally controlled by medication and who do not have dementia is deep brain stimulation (DBS). In DBS, electrodes implanted into the subthalamic nucleus or inner part of the globus pallidus provide electrical stimulation to these and surrounding structures. This stimulation induces physiological and chemical changes that ameliorate some of the motor symptoms of PD by a poorly understood mechanism. Unilateral tremor and rigidity may also respond to stereotactic ablation of the contralateral globus pallidus, ventrolateral thalamus, or subthalamic nucleus.
DIFFUSE LEWY BODY DISEASE
Diffuse Lewy body disease (DLBD) (Lewy body dementia)is a sporadic neurodegenerative disease, which is thought to be the second most common cause of dementia after AD. It combines the neurological manifestations of dementia and parkinsonism. Unlike AD in which short term memory is affected early, patients with DLBD present with fluctuating attention and cognition and visual hallucinations. Motor parkinsonian manifestations (bradykinesia, rigidity, and less frequently tremor) vary in severity and may appear later. DLBD patients have also depression, sleep disorder, and autonomic dysfunction. The brain in DLBD is not as atrophic as it is in AD, and shows small, inconspicuous Lewy bodies in the neocotex, limbic system, and brainstem. A large proportion of DLBD patients also have AD pathology.
MPTP PARKINSONISM
The pyridine analogue MPTP (1-methyl-1-4-phenyl-1,2,3,6-tetrahydropyridine) is taken up selectively by dopaminergic neurons. Its active compound, MPP (1-methyl-4-phenylpyridinium) inhibits mitochondrial function and induces cell death. Toxic damage of dopaminergic neurons causes parkinsonian symptoms. This was discovered when a drug addict accidentally injected himself with synthetic heroin made by himself, contaminated by MPTP. MPTP parkinsonism in humans and experimental animals resembles PD. Low dose intravenous infusion of the pesticide (and herbicide) rotenone, an inhibitor of complex I of the mitochondrial respiratory chain, causes degeneration of the SN and Lewy body-like inclusions in rats. The MPTP model, the rotenone experiments, and the ALS- Parkinson-Dementia cases from Guam (see below) suggest that environmental neurotoxins play a role in the pathogenesis of PD.
GUAM ALS-PARKINSON-DEMENTIA (GPD)
A combination of amyotrophic lateral sclerosis, PD and dementia is frequent among the Chamorro people of Guam. GPD is a combination of tauopathy and synucleinopathy. NFTs are seen in the cortex and subcortical nuclei, including the SN, and α-synuclein inclusions are present in the entorrhinal cortex, amygdala, and SN. The appearance of this disease in successive generations implicates genetic factors. However, in recent years, the incidence of GPD has declined dramatically. Epidemiological work and animal experiments support the hypothesis that GPD is caused by a toxic amino acid in the seed of a cycad plant which is used to make flour and is a staple in the local diet. Another hypothesis implicates other environmental factors leading to deficiencies of calcium and magnesium, and high concentrations of aluminum and other minerals in drinking water, with aluminum deposition in neurons. Aluminum can disrupt the neuronal cytoskeleton and cause neurofibrillary pathology. The decline of GPD in recent years has been attributed to changes in diet and improved nutrition. GPD underlines the important role of genetics and environmental neurotoxins in the pathogenesis of neurodegenerative diseases.
Parkinsonian syndromes may also develop in the course of other conditions that damage the SN, e.g., striatonigral degeneration, postencephalitic parkinsonism, manganese poisoning, carbon monoxide poisoning, hypoxic-ischemic encephalopathy, traumatic brain injury, and stroke.
Further Reading
- Houlden H, Singleton A B. The genetics and neuropathology of Parkinson's disease. Acta Neuropathol 2012;124:325–338.PubMed
- Okun M S Deep-Brain Stimulation for Parkinson's Disease. N Engl J Med 2012; 367:1529-1538.PubMed
- Migdalska-Richards A, Schapira AH. The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem 2016;139 Suppl 1:77-90. PubMed
- Lill CM. Genetics of Parkinson's disease. Mol Cell Probes. 2016;30:386-396.PubMed
- Diederich NJ, Uchihara T, Grillner S, and Goetz CG. The Evolution-Driven Signature of Parkinson’s Disease. Trends Neurosci 2020 Jul;43(7):475-492.PubMed
Updated: February, 2021