Fungal, Protozoan and Parasitic Infections of the Nervous System
The most common CNS mycoses are, in order of frequency, Candidiasis, Aspergillosis, and Cryptococcosis. They are seen mainly in patients with AIDS and other immunosuppressed states.
Candida is also frequent in the neonatal period. Candida is a commensal but rarely causes disease in normal people. Infection is caused by organisms that are already present in the intestine and other locations, and in neonates it is trasmitted from external sources. Most disseminated infections are nosocomial, and the key risk factors are catheters, and antibiotics. Candida consists of budding yeasts and hyphae. It causes meningitis, multiple microabsesses, or extensive brain necrosis. At first, the inflammation consists of neutrophils; later of epithelioid cells and giant cells.
Aspergillus and the related soil fungus mucor (rhizopus) are branching hyphae. They are ubiquitous in nature but only cause disease in immunosuppressed persons. The most important risk factors for disseminated infection are neutropenia, cytotoxic chemotherapy, and corticosteroids. Aspergillus enters the body through the lungs. Mucor, which is also common in patients with diabetic ketoacidosis, infects the nasal mucosa from where is spreads to the brain. Both fungi have the tendency to invade blood vessels and cause thrombosis with cerebral infarction or vascular rupture with cerebral hemorrhage.
The angioinvasive properties of aspergillus are due to the production of elastase by some species of this fungus.
Cryptococcus
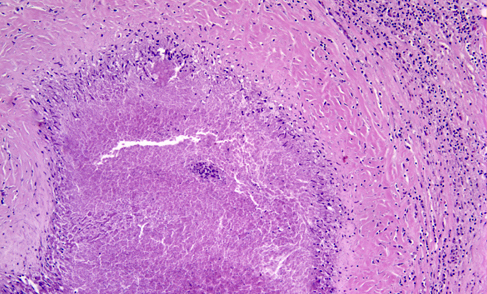
Cryptococcus is an oval yeast about the size of a red cell, surrounded by a gelatinous capsule. There are several species of cryptococcus. The most common, cryptococcus neoformans, causes 95% of infections. It is a worldwide fungus, present in bird droppings, vegetables, and soil. It may affect healthy individuals but is particularly common in immunodeficient patients, especially patients with HIV/AIDS. It is most often community-acquired. The portal of entry is the respiratory tract but pulmonary infection may be asymptomatic or minimally symptomatic. Cryptococcus spreads to the brain from the lungs and often causes meningitis without involving other organs. In the CNS, cryptococcus grows extensively in the subarachnoid space and perivascular spaces, which become cystically distended to the point that brain sections look like Swiss cheese. In immunosuppressed individuals, inflammation is absent or mild. In immunocompetent hosts, cryptococcus elicits a cell-mediated immune reaction with lymphocytes and epithelioid cell granulomas. Rarely cryptococcus may cause mass lesions (cryptococcomas). Cryptococcus meningitis has an insidious onset and may go on from weeks to years. It can cause hydrocephalus, dementia, cranial neuropathies and focal neurological deficits. The CSF in cryptococcosis shows mononuclear pleocytosis, elevated protein, and low glucose, similar to tuberculous meningitis. Yeasts can be identified by microscopy of the CSF and their antigens can be detected by latex agglutination.
Coccidioidomycosis
The soil fungus coccidioides, which is endemic to the southwestern United States (especially the San Joakim Valley of California), Mexico and parts of Central and South America, can cause disseminated infections including meningitis. Coccidioidal meningitis affects most commonly young men and causes headache, cognitive decline, personality changes, visual disturbances, gait abnormality and focal neurological deficits. African Americans and immunocompromised persons are at increased risk. Patients with CNS coccidioidomycosis have a positive serum coccidioidal antibody test. CSF analysis shows lymphocytic and eosinophilic pleocytosis, markedly elevated protein and low glucose. Untreated CNS coccidiomycosis is fatal. Autopsy shows a basal meningitis, ventriculitis, hydrocephalus, cerebritis and arteritis with infarcts. The inflammatory infiltrate is granulomatous and contains coccidioidal spherules with endospores.
Toxoplasmosis
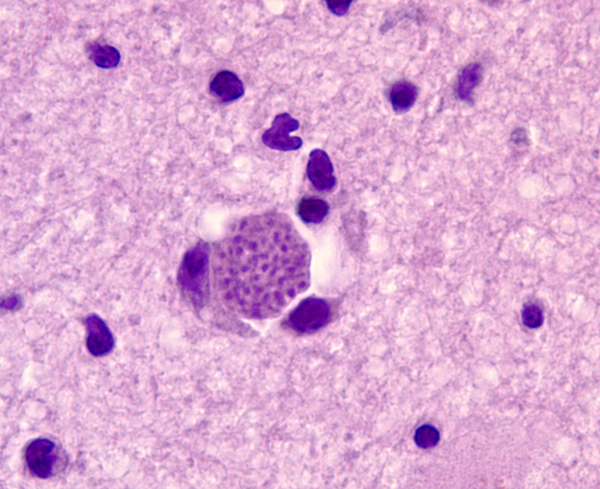
The protozoon Toxoplasma gondii infects approximately one-third of the world’s population. The organism reproduces sexually in the intestinal tract of cats and forms oocysts that are excreted in cats’ feces. Protozoa released form oocysts ingested by animals and humans invade the brain, eye, heart, skeletal muscle, and other organs, where they form tissue cysts. Humans and animals are infected from oocysts in cat feces and from poorly cooked meat containing tissue cysts. Parasites released from oocysts and tissue cysts reside in cell vacuoles and are protected from host defenses. Most primary infections are asymptomatic or cause a self-limited granulomatous lymphadenitis. Latent infections are also silent. If the primary infection occurs during pregnancy, toxoplasma may cross the placenta and cause a devastating necrotizing encephalitis and chorioretinitis in the fetus. The end result of congenital toxoplasmosis is severe brain damage, microcephaly, cerebral calcifications, and blindness. Reactivation of latent toxoplamosis in immunosuppressed individuals, such as patients with HIV/AIDS, causes toxoplasma encephalitis, characterized by necrosis and mononuclear cell infiltrates. The lesions contain single organisms and cysts, which can be identified histologically or by immunohistochemistry. About 25% of patients dying from AIDS have toxoplasma encephalitis.
Recent clinical studies have demonstrated an association between latent CNS T. gondii infection, schizophrenia, and suicide. The presumed mechanism of mental illness is neuroimmunological disturbance stemming from the presence of T. gondii in the brain.
Cysticercosis
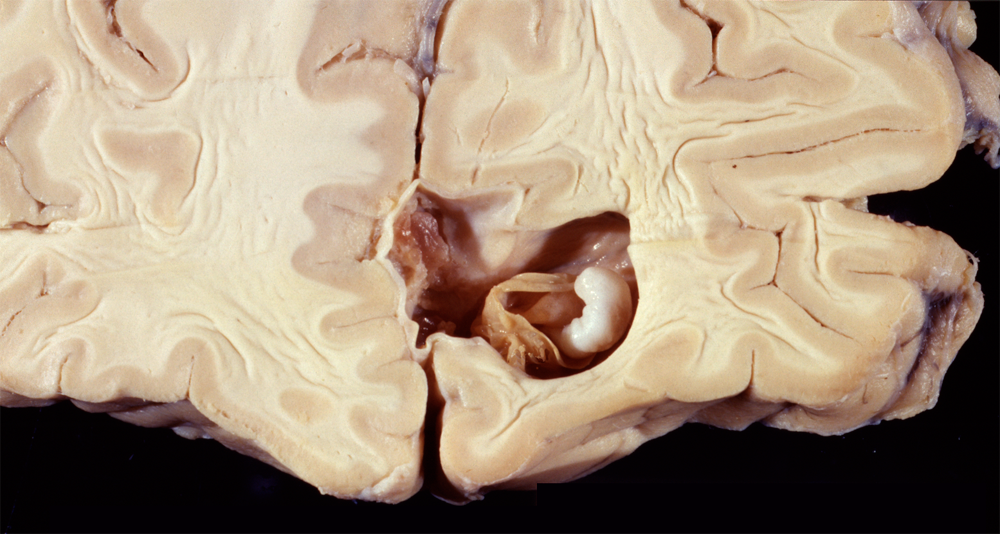
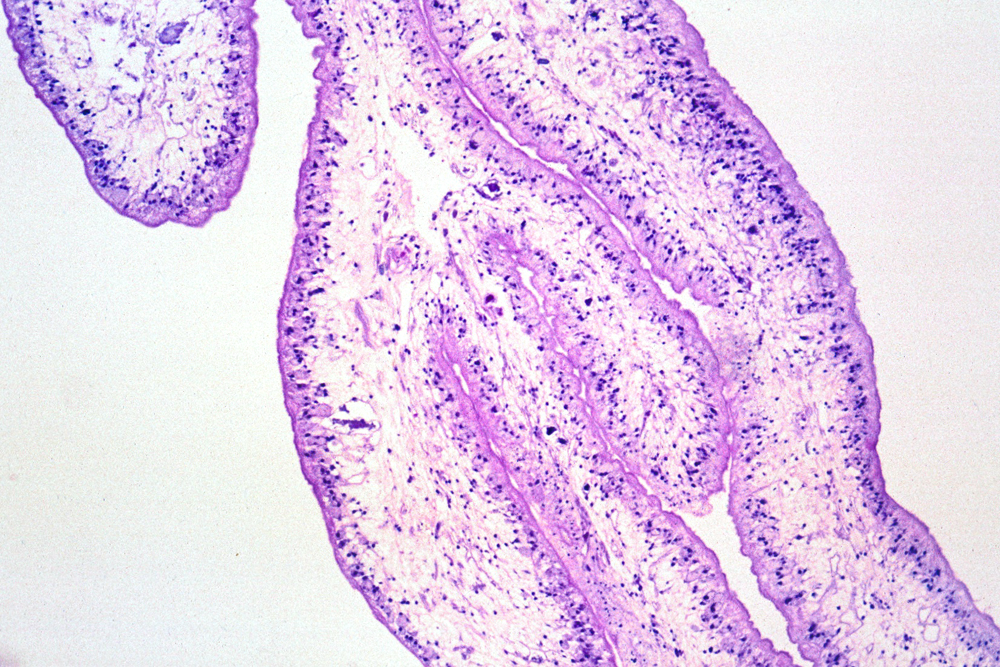
Cysticercosis is the most common parasitic infection and neurocysticercosis is the most common cause of secondary seizures worldwide. Cysticercosis is caused by ingestion of water, vegetables and other foods fecally contaminated by eggs excreted by carriers of the pork tapeworm, Taenia Solium. In the intestine, the eggs transform into oncospheres which penetrate the intestinal wall, enter the circulation and lodge in the brain, eyes, muscles and other tissues. There, oncospheres develop into cysticerci. The same sequence occurs in pigs, which are the intermediate host of T. solium. In pigs, cysticercosis affects primarily muscles. After consumption of pork containing cysticerci, scolices attach to the small intestinal wall and develop into adult worms. This condition is called intestinal taeniasis. Eggs or proglotids of the adult worms are then shed in the feces, contaminating the environment.
Further Reading
- Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii Infection and Self-directed Violence in Mothers. Arch Gen Psychiatry. 2012 Jul 2:1-8. doi: 10.1001/archgenpsychiatry.2012.668. [Epub ahead of print] PubMed
Updated: January, 2022.