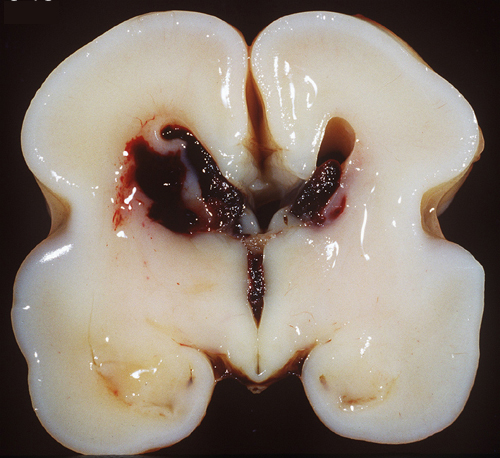
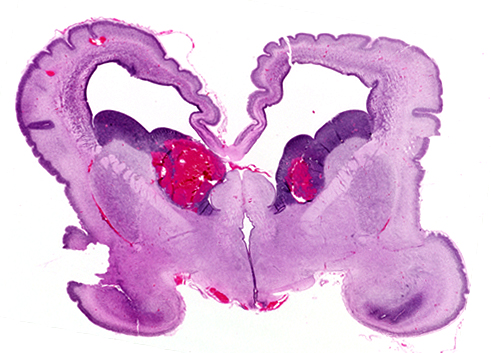
GERMINAL MATRIX AND INTRAVENTRICULAR HEMORRHAGE
Germinal matrix hemorrhage (GMH) is a frequent lesion in premature babies who also have hyaline membrane disease and the respiratory distress syndrome.In the 1970s, the frequency of GMH in premature neonates was 50%. By the 1980s, its incidence in newborns weighing less than 1500 gm dropped to 20% and has not changed. It is a major cause of morbidity and mortality in the newborn period and of cerebral palsy and mental retardation later on. GMH usually develops 24 to 48 hours after birth, but it can occur at any time, including prenatally. most cases are clinically silent.
The germinal matrix is a thick cellular layer of immature cells (neuronal and glial precursors) under the ependymal lining of the ventricles. This layer is traversed by a rich network of delicate vessels that have no structural support and lack autoregulation, which makes them susceptible to fluctuations of blood flow. In babies with hyaline membrane disease, these vessels dilate because of passive congestion, their walls are damaged because of hypoxia, and they rupture. The hemorrhage starts usually between the thalamus and the caudate nucleus, adjacent to the foramina of Monro, where the germinal matrix is thicker, and is frequently bilateral. If it is large, it ruptures into the ventricles, flooding the lateral, third, and fourth ventricles (intraventricular hemorrhage - IVH). Blood then exits through the foramina of Luschka, causing subarachnoid hemorrhage. Thick clots along the ventral aspect of the brain stem may block the foramina of Luschka. Four grades of GMH are distinguished by imaging: grade I (confined to the germinal matrix), grade II (intraventricular hemorrhage without ventricular dilatatation), grade III (intraventricular hemorrhage with ventricular dilatation), and grade IV (GMH with intraventricular rupture and hemorrhage into the surrounding white matter). Grade I and II GMH have few or no clinical effects. Grade III and IV IVH have severe complications.
Large, bilateral IVH causes fatal acute distention of the ventricles or exsanguination into the ventricles and subarachnoid space. Patients surviving large IVH often develop hydrocephalus due to clots or gliosis of the aqueduct and from obliteration of the foramina of Luschka and subarachnoid space by clots and the fibrous tissue that develops from their organization.
 Post-hemorrhagic hydrocephalus |
 Post-hemorrhagic hydrocephalus |
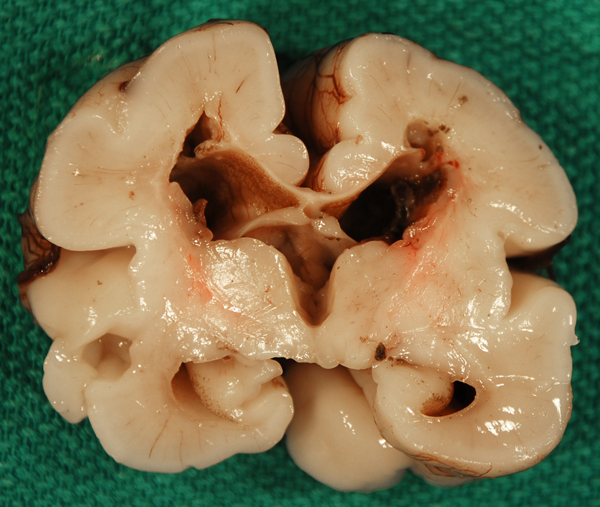 Periventricular white matter infarction, old |
Another complication is periventricular white matter infarction, which is thought to be a venous infarct of the white matter adjacent to the GMH caused by compression of terminal veins by the hemorrhage. This may cavitate, causing porencephaly, and correlates with the spastic hemiparesis that is seen following some cases of GMH. Neurogenesis and neuronal migration are completed by midgestation. Therefore, GMH has little effect on brain development, unless it occurs early in the second trimester, in which case it may potentially deplete the pool of neuronal and glial precursors.periventricular white matter infarction, which is thought to be a venous infarct of the white matter adjacent to the GMH caused by compression of terminal veins by the hemorrhage. This may cavitate, causing porencephaly, and correlates with the spastic hemiparesis that is seen following some cases of GMH. Neurogenesis and neuronal migration are completed by midgestation. Therefore, GMH has little effect on brain development, unless it occurs early in the second trimester, in which case it may potentially deplete the pool of neuronal and glial precursors.
The causes of GMH are multiple. Local anatomical factors include the high vascularity of the germinal matrix, its immature fragile capillary bed with poor stromal support, and the sharp U-turn the thalamostriate veins take at that point, which makes the germinal matrix prone to congestion. Systemic factors include capillary damage from hypoxia, loss of vascular autoregulation, fluctuations in blood flow velocity, and venous congestion. The pathogenesis of GMH probably involves an interaction between the environmental factors listed above and genetic variations affecting inflammatory pathways, coagulation, and vacular structure. Muscle paralysis eliminates fluctuations of cerebral blood flow velocity and reduces the incidence of IVH.
The germinal matrixes of the immature cerebellum are the external granular layer and the subependymal layer of the roof of the 4th ventricle. Hemorrhage within these layers, once considered rare, is being increasingly recognized by neonatal ultrasound and more by MRI, especially in extremely low birth weight infants and contributes to their already high morbidity and mortality. The causes of cerebellar hemorrhage include the same systemic factors that are involved in IVH.
Further Reading
- Papile LA, Burstein J,Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants withbirth weight less than 1,500 gm. J Pediatr 1978;92:529-34. PubMed
- Woodward LJ, Andeson PJ, Austin NC et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685-94. PubMed
- Limperopoulos C, Benson CB, Bassam H, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 2005;116:717-24. PubMed
- Ballabh P.Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 2010;67:1-8.PubMed
- Ment LR, Ådén U, Lin A, et al. Gene-environment interactions in severe intraventricular hemorrhage of preterm neonates. Pediatr Res 2014;75:241-50.PubMed
- . Gilard V, Tebani A, Bekri S, Marret S. Intraventricular Hemorrhage in Very Preterm Infants: A Comprehensive Review. J Clin Med 2020 Jul 31;9(8):2447. doi: 10.3390/jcm9082447.PubMed .